KRISS Develops Dried Blood Spot Certified Reference Materials for Newborn Screening
- Writerkrissadmin
- Date2022-10-25 00:00
- Hits1076
KRISS Develops Dried Blood Spot Certified Reference Materials for Newborn Screening
- First Certified Reference Materials targeting diagnostic markers of inherited metabolic disorders in newborn screening -
- Enhanced reliability of dried blood spot samples expected to expand remote diagnosis applications -
# A’s baby underwent a newborn screening test for inherited metabolic disorders within seven days of birth. The test checks for risk factors such as hypothyroidism, phenylketonuria, maple syrup urine disease, which can lead to developmental disabilities if not detected in their early stage. Every year, one in every 1,000 newborns are diagnosed with inherited metabolic disorders.
The Korea Research Institute of Standards and Science (KRISS, President Hyun-Min Park) has developed Certified Reference Materials (CRMs)* that can enhance the reliability of using dried blood spot testing for newborn screening.
* CRM: A reference material that serves as a standard in determining the accuracy of measurements and analytical methods

▲ KRISS Biodiagnostics Analysis Team
(From left: Seohyun Choi, master’s student of UST-KRISS School; Ji-Seon Jeong, principal researcher; Ha-Jeong Kwon, principal researcher; Nordiana Rosli, doctoral student of UST-KRISS School)
DBS is a sample obtained by drying a drop of blood from the finger or heel on a piece of filter paper. This approach is used for screening rather than actual diagnosis, as it is less accurate than venous blood sample tests. Its common applications include newborn screening for inherited metabolic disorders and doping control during Olympics.
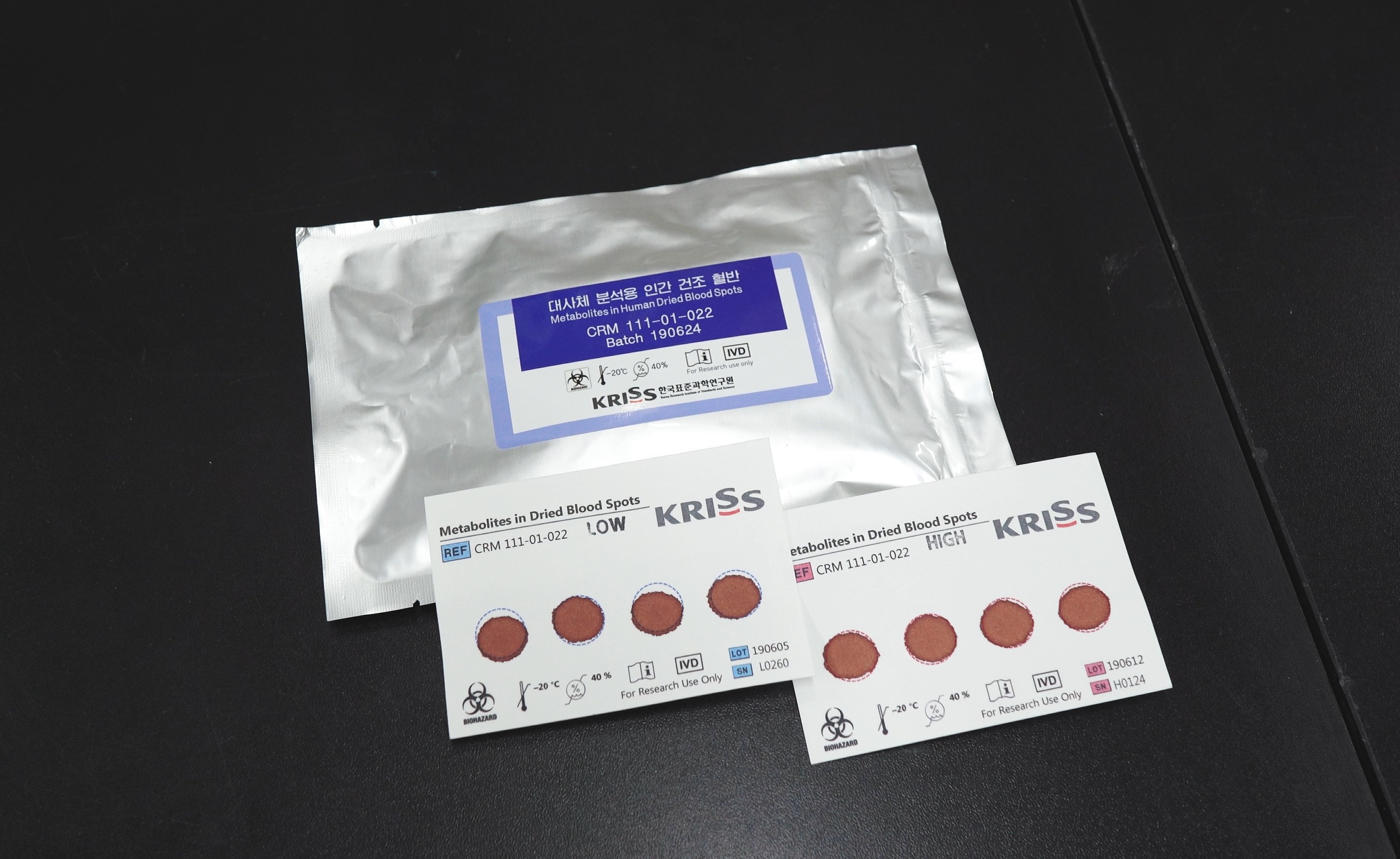
▲ DBS CRM for metabolites analysis
The proposed CRM provides eight certified values and 10 reference values for amino acids, glucose, galactose, and acylcarnitines, which are diagnostic markers of inherited metabolic disorders in newborns. This allows accurate measurement of the amount of target compounds in the DBS.
▲ Overview of development of DBS CRMs
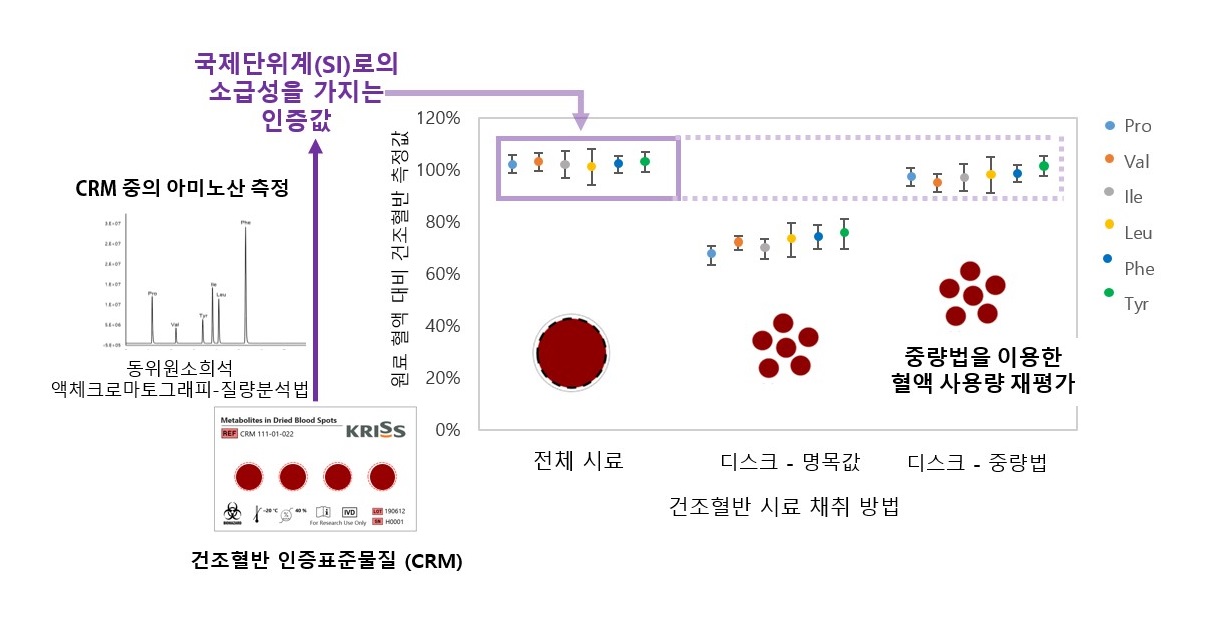
The lack of reference values has made it difficult for DBS testing to be considered reliable for medical decision. In addition, there has been a problem with measurement bias caused by the need to retrieve portions of blood spots using a paper puncher.
The KRISS Biodiagnostics Analysis Team found that a 0.4 mm bias in diameter led to a 0.78 μL (one millionth of a liter) difference in sample volume.

▲ A researcher is fabricating the proposed DBS CRM for metabolites analysis.
The research team controlled the sample volume to 50 μL during the CRM manufacturing stage, and proposed bias-free measurements as certified values, thereby successfully creating CRMs with complete measurement traceability to the International System of Units. This is the first-ever development of DBS CRMs.
Dr. Ji-Seon Jeong, a principal researcher at KRISS, said, “DBS has come under the spotlight as a convenient way of blood sampling, which satisfies the high demand for remote healthcare and home sampling in the days of the pandemic. Our study has laid the foundation to improve the reliability in DBS sample measurement, opening the door for DBS to become an effective tool not only in screening but also diagnosis.”
KRISS plans to develop more CRMs for other diagnostic markers used in newborn screening.
Funded by KRISS, the study was published in the world-leading journal Analytical Chemistry (IF: 8.008) in July.

