
News & Updates
Determining “the Temperature at the Moment” When a Liquid Starts Freezing
- Writerkrissadmin
- Date2018-05-24 00:00
- Hits11462
Determining “the Temperature at the Moment” When a Liquid Starts Freezing
- The First and Only Success in the world in realizing the ideal standard temperature -
Principal Researcher Dr. Wukchul Joung at the Center for Thermometry and Fluid Flow Metrology, KRISS has successfully determined the liquidus temperature of a fixed-point metal (i.e. tin), which is considered as an ideal reference temperature in the international temperature standard, by using a novel temperature control technique. He has also revealed that there is a huge difference between the freezing temperature of tin, which is used as the current reference temperature for 231.928 ℃ in the International Temperature Scale of 1990 (ITS-90), and the determined liquidus temperature of tin. This is the first case in the world where the standard temperature that has been believed to exist only ideally is actually realized and determined.
* Liquidus Temperature: the temperature at which a molten sample with a homogeneous impurity concentration begins to freeze (or finishes melting), and with a specific impurity concentration, the liquidus temperature is uniquely determined.
Each substance has four phase-change temperatures, such as the freezing temperature, the melting temperature, the solidus temperature, and the liquidus temperature. If the substance is pure, all these phase-change temperatures have the same value, and the temperature during the phase change (i.e. melting or freezing) remains constant.
? International Temperature Scale of 1990 (ITS-90) defines temperature in the range from –259.3467 ℃ to 961.78 ℃ using the phase-change temperatures of 12 selected substances as the reference temperatures (i.e. fixed-point temperatures). Phase-change temperatures of metals are used for most of the temperature range while those of gases are used for cryogenic temperatures.
However, there is not a pure substance in the real world. Impurities in those substances change the phase-change temperature during freezing or melting; thus a range of the phase-change temperature develops and the four phase-change temperatures will always, in practice, have different values. In this circumstance, the only value, which is unique and invariable, is the liquidus temperature. Even though the temperature changes during the freezing or melting process, the temperature, at which freezing starts (or melting finishes), is always unique. Therefore, the ITS-90 considers the liquidus temperature of the fixed-point substances as the most ideal reference temperatures in the temperature scale.
The problem was that there was no proper methods to realize and determine the liquidus temperature of the substances having (trace amount of) impurities. Especially for metals, which have relatively high phase-change temperatures, it was almost impossible to determine the temperature, at which the freezing starts (or the melting finishes), due to the deep temperature depression at the start of freezing (i.e. supercooling) or the rapid temperature increase near the end of the melting (i.e. run-off).
Alternatively, as an indirect method, the freezing or melting temperatures of the fixed-point metals have been used so far as the best approximation to the liquidus temperature. However, because of the existence of impurities, these temperatures cannot satisfy the basic requirements for the reference temperature; that is, the uniqueness and the invariability.
To solve this problem, Dr. Wukchul Joung has realized and determined the liquidus temperature of tin, a fixed-point metal defining 231.928 ℃, by the heat pulse-based melting method using the hydraulic temperature control technique, which was originally developed at KRISS and is capable of controlling temperature in unsurpassable speed and precision.
The liquidus temperature of tin determined this time was about 0.00095 ℃ (i.e. 0.95 mK) higher than the current freezing temperature of tin; this deviation was clearly beyond the internationally acceptable range of deviation in terms of the temperature standard.
This proved that there is a huge difference between the freezing temperature of tin and the liquidus temperature of tin which is the ideal reference temperature. In addition, the findings of the research can be highly valued given that the research has successfully analyzed and proved the influence of the trace amount of impurities on the phase-change temperatures of a substance, which had not clearly established thus far, and the findings can lead to revision of the future International Temperature Scale (ITS-XX).
Dr. Joung said, “Thanks to the achievements the research team has made, the reference temperature of the ITS-90 has become more precise and accurate.” He added, “It has a special meaning in that KRISS has overcome technical difficulties, which the international society has struggled, and has become the first and the only national metrology institute (NMI) in the world that realizes the ideal temperature standard.”
The findings of this research were published in two papers in the latest version of the internationally renowned journal of measurement science, Metrologia (IF: 3.411).
○ Explanation of “determination of the liquidus temperature of tin”
The liquidus temperature is the temperature at which a molten sample with a homogeneous impurity concentration begins to freeze (or finishes melting), and with a homogeneous impurity concentration and at a particular pressure around the sample, the liquidus temperature is uniquely determined. However, it is not possible to measure the liquidus temperature in typical freezing or melting processes, because of the deep temperature decrease at the start of freezing (i.e. supercooling) or the rapid temperature increase near the end of the melting (i.e. run-off).
Therefore, the liquidus temperature should be determined in such a way as to find the temperature at the end of the melting (which equals the start of the freezing) by melting the sample with controllable heat pulses, measuring the melting temperature at specified melted fractions, and extrapolating the temperature to the end of melting.
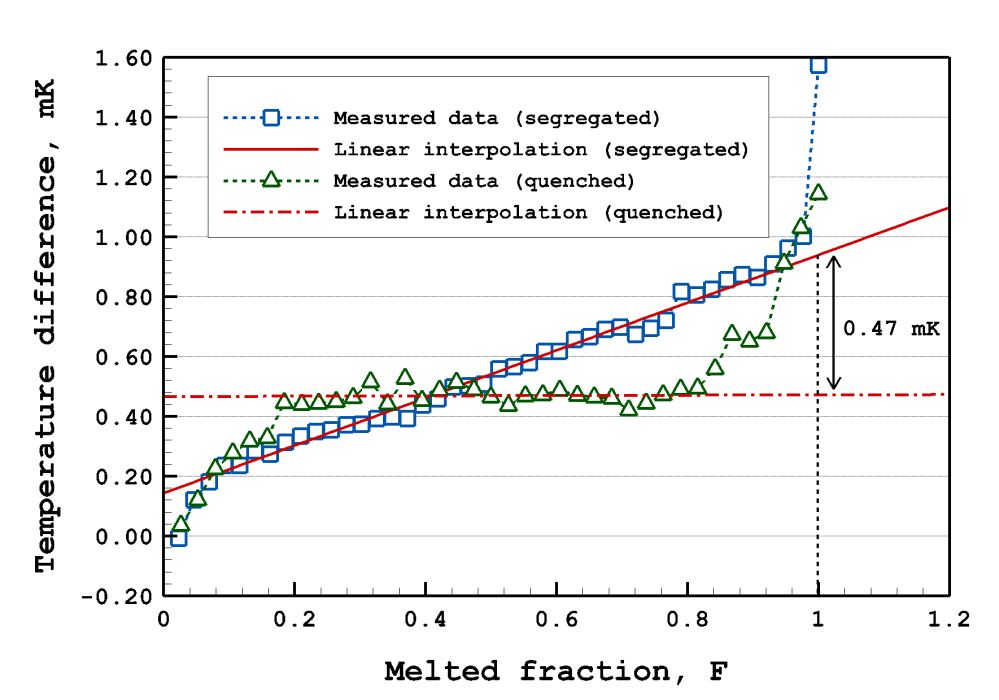
▲ [Picture] Liquidus Temperature Determination Process through Precise Control of the Phase Change Speed of Metal (Tin)
[Picture] shows the measured melting temperatures at specific melted fractions (F) during the heat pulse-based melting which was performed by applying discrete temperature pulses to the frozen sample. From the melting temperatures at various melted fractions, the liquidus temperature was determined by extrapolating the temperature to the end of the melting.
This result also showed that melting temperature could vary in different manners depending on the impurity distributions inside of sample (e.g. blue square and green triangle marks in [Picture2]). In particular, it was first proved that the liquidus temperature (red solid line at F = 1) or solidus temperature* (red dot-and-dash line at F = 1) could be determined by distributing the impurities in a controlled manner and melting the sample by the heat pulse-based melting.
*solidus temperature: the temperature at which freezing finishes or melting starts.
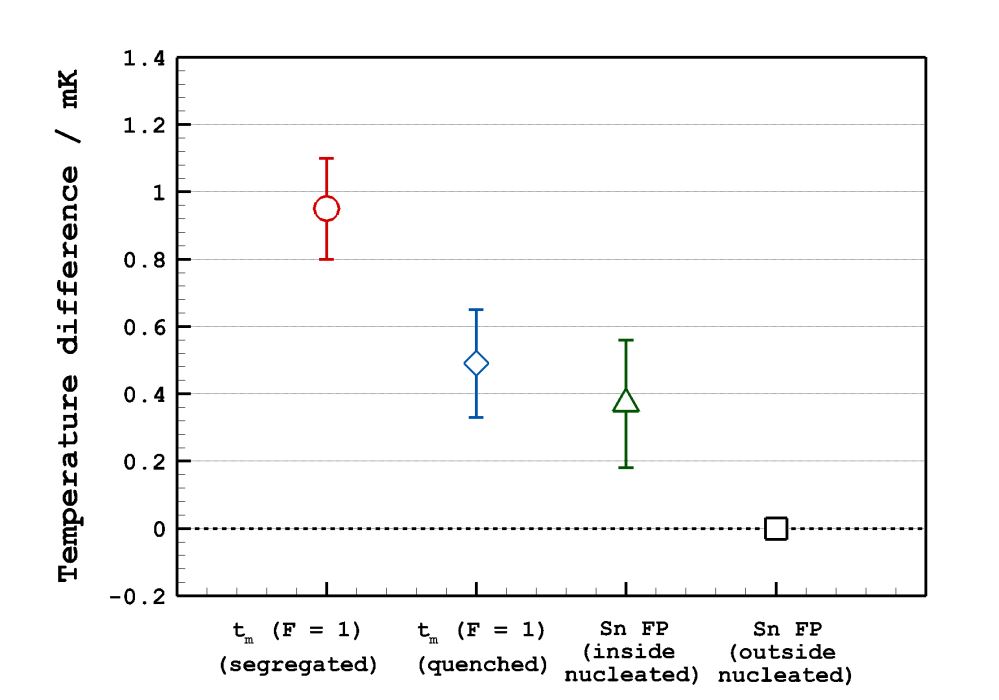
▲ [Picture 3] Temperature Differences between the Liquidus Temperature of Tin and the Current Freezing Temperatures of Tin

▲ Dr. Joung is realizing the liquidus temperature of tin by using the hydraulic temperature control apparatus.
QUICK MENU 원하시는 서비스를 클릭하세요!
등록된 퀵메뉴가 없습니다.
